Practice Test: Question Set - 01
1. In the reaction, Ca + 2H2O = Ca(OH)2 + H2; what volume (c.c.) of hydrogen at STP would be liberated, when 8 gm of calcium reacts with excess water ? (Atomic weight of calcium = 40).
- (A) 4480
- (B) 2240
- (C) 1120
- (D) 0.4
2. A vapor whose partial pressure is less than its equilibrium vapor pressure is called a __________ vapor.
- (A) Saturated
- (B) Supersaturated
- (C) Superheated
- (D) None
of these
3. Dissolving a solute in a solvent does not change its
- (A) Specific
heat
- (B) Vapor
pressure
- (C) Viscosity
- (D) None
of these
4. Applicability of Clausius-Clapeyron Equation is subject to the condition that the
- (A) Vapor
follows ideal gas law
- (B) Volume
in the liquid state is negligible
- (C) Both (a)
& (b)
- (D) Neither (a)
nor (b)
5. 'Cox' chart which is useful in the design of a distillation column (particularly suitable for petroleum hydrocarbons) is a plot of the
- (A) Temperature
vs. log (vapor pressure)
- (B) Vapor
pressure vs. log (temperature)
- (C) Log
(temperature) vs. log (vapor pressure)
- (D) Vapor
pressure vs. temperature
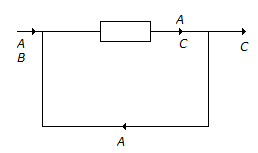
6. The reaction A + B → C has been conducted in a reactor as shown below. The number of boundaries around which material balance can be written, are
- (A) 1
- (B) 6
- (C) 3
- (D) 4
7. Methane is mixed with stoichiometric proportion of oxygen and completely combusted. The number of additional specifications required to determine the product flow rate and composition is
- (A) 0
- (B) 1
- (C) 2
- (D) 3
8. The number of water molecules present in a drop of water weighing 0.018 gm is 6.023 × __________.
- (A) 1026
- (B) 1023
- (C) 1020
- (D) 1019
9. Concentration of a solution expressed in terms of ____________ is independent of temperature.
- (A) Molarity
- (B) Normality
- (C) Molality
- (D) None of
these
10. The amount of Zn (atomic weight = 65) required to form 224 c.c. of H2 at N.T.P. on treatment with dilute H2SO4 will be __________ gm.
- (A) 0.065
- (B) 0.65
- (C) 6.5
- (D) 65
11. Pick out the correct conversion.
- (A) 1 BTU = 453.6
calories
- (B) 1 BTU = 252
calories
- (C) 1 calorie =
252 BTU
- (D) 1 calorie =
453.6 BTU
12. A vapor that exists above its critical temperature is termed as a __________ vapor.
- (A) Saturated
- (B) Unsaturated
- (C) Gaseous
- (D) Sub-cooled
13. Othmer chart is useful in estimating the heat of
- (A) Mixing
- (B) Wetting
- (C) Adsorption
- (D) None of
these
14. Pick out the wrong unit conversion of temperature.
- (A) °R = 273 +
°F
- (B) Temperature
difference of 1°K = 1°C = 9/5°F
- (C) °C = (F- 32)
× 0.555
- (D) °F = (°C +
17.778) × 1.8
15. Pick out the wrong unit conversion of heat transfer rate.
- (A) 1 kcal/hr=
1.163 Watt
- (B) 1 Watt =
1.163 kcal/hr
- (C) 1 BTU/ft2.hr
= 2.712 kcal/m2.hr
- (D) 1 kcal/m2.hr
= 0.3687 BTU/ft2.hr = 1.163 Watt/m2