Practice Test: Question Set - 01
1. In case of staged packed bed reactors carrying out exothermic reaction, use
- (A) High recycle
for pure gas
- (B) Plug flow
for dilute liquid requiring no large preheating of feed
- (C) Cold
shot operations for a dilute solution requiring large preheating to bring the
stream upto the reaction temperature
- (D) All (a), (b)
and (c)
2. The performance equations for constant density systems are identical for
- (A) P.F.R.
and backmix reactor
- (B) P.F.R.
and batch reactor
- (C) P.F.R.
batch reactor and backmix reactor
- (D) Batch
reactor and backmix reactor
3. The equilibrium constant of chemical reaction __________ in the presence of catalyst.
- (A) Increases
- (B) Decreases
- (C) Remain
unaffected
- (D) Can either
increase or decrease (depends on the type of catalyst)
4. Study of chemical kinetics is the easiest in the case of __________ reactions.
- (A) Irreversible
- (B) Reversible
- (C) Surface
- (D) Side
5. With increase in temperature, the equilibrium conversion of a reversible exothermic reaction
- (A) Decreases
- (B) Increases
- (C) Remain
unaffected
- (D) Decreases
linearly with temperature
6. The point selectivity of the product 'Y' in the reaction as shown in the bellow figure, is equal to
- (A) K1/K2
- (B) K2/K1
- (C) K1
- K2
- (D) K2
- K1
7. For reaction, P + 2 → 3R, molar rate of consumption of P is
- (A) Double of
that of Q
- (B) Same
as that of Q
- (C) Half of that
of Q
- (D) 2/3rd of that
of Q
8. Pick out the wrong statement.
- (A) Visible
radiation provides the necessary activation energy in photochemical reactions
- (B) The
order and molecularity of a complex reaction may not be the same
- (C) For a second
order reaction, the slope of the graph/plot between rate and (concentration) is
equal to the rate constant (k)
- (D) Molecularity
of the reaction is always a whole number greater than zero
9. In a first order reaction, the time required to reduce the concentration of reactant from 1 mole/liter to 0.5 mole/liter will be __________ that required to reduce it from 10 moles/liter to 5 moles/liter in the same volume.
- (A) More than
- (B) Less than
- (C) Same as
- (D) Data
insufficient; can't be predicted
10. An irreversible first order reaction is being carried out in a CSTR and PFR of same volume. The liquid flow rates are same. The relative conversion will
- (A) Be more in
CSTR than in PFR
- (B) Be more in
PFR than in CSTR
- (C) Be same in
both cases
- (D) Depend
on the temperature
11. The first order series reaction as shown in the bellow figure is conducted in a batch reactor. The initial concentrations of A, B and C (CA0, CB0, CC0 respectively) are all non-zero. The variation of CB with reaction time will not show a maximum, if
- (A) k2 CB0 > k1 CA0
- (B) k CA0 > k2.CB0
- (C) CB0 > CA0
- (D) CA0 > CB0
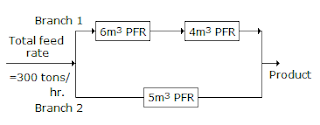
12. Three plug flow reactors (PFR's) of 4, 5 & 6 m3 volumes are arranged in two branches as shown below in the figure.
If the total feed rate is 300 tons/hr, then for the same conversion in each branch, the feed rate through branch II should be __________ tons/hr.
- (A) 100
- (B) 150
- (C) 200
- (D) 225
13. Participation of __________ is involved in the occurrence of a chemical reaction.
- (A) Protons
- (B) Neutrons
- (C) Electrons
- (D) None of
these
14. The first order gas phase reaction as shown in the bellow figure is conducted isothermally in batch mode. The rate of change of conversion with time is given by
- (A) dXA/dt = k1 (1 - XA)2 (1 + 2XA)
- (B) dXA/dt = k1 (1 - XA) (1 + 0.5XA)
- (C) dXA/dt = k1 (1 - XA)
- (D) dXA/dt = k1 (1 - XA)/(1 + XA)
15. A photochemical reaction is __________ light.
- (A) Initiated by
- (B) Accompanied
with emission of
- (C) Catalyzed by
- (D) Used
to convert heat energy into